WHAT ARE INDUSTRIAL CATALYSTS?
Catalysts are of great importance to a series of manufacturing industries, ranging from food manufacturing industries to petroleum industries. They aid in industrial refining and chemical production processes.
They help to save energy. They also play an important role in developing new sources of energy. The usefulness of catalysts in the industrial world is vital. Catalysts make chemical production processes safer, easier, and faster. They accomplish this by regulating the activation energy required to start chemical reactions.
Catalysts make the production of various materials quicker, easier, more efficient. They reduce the waste generated during manufacturing processes. These are all important to industries as it makes production rates faster.
Another important aspect of catalysts is that they're necessary to produce specific products. In these situations, the catalyst performs a dual function, where it not only acts as an accelerator or a decelerator, but also acts as a recoverable reactant.
Various environmental pollution laws have contributed to the value of catalysts and brought about an increase in demand for catalysts by researchers and manufacturers. Catalysts make industrial production processes more environmentally friendly. A prominent example is refining catalysts used in petroleum refineries as opposed to the traditional thermal cracking process, which generates quite a bit of environmental pollution.
Scientific advances led to the discovery of new products. New products use catalysts in their production processes; hence, there even more of a demand for catalysts.
These desirable qualities that catalysts possess, and the increasing demand for catalysts, have made it worthwhile for chemists and scientists at large to research and discover new catalysts. Chemists and scientists also research how catalysts can be produced in larger quantities to meet the rising demand. They're also interested in ways by which catalysts can be made more efficiently.
As crucial as catalysts are, they can also be harmful to us and our environment if they are not treated or disposed of properly after their catalytic properties have spent.
What Are Industrial Catalysts?
In 1746, J. Hughes used nitrogen oxide catalysts in the production of sulfuric acid in large quantities. This was the first time catalysts were used in large amounts on an industrial scale.
A catalyst is a substance that alters the rate of chemical reactions without being part of the product. In the sense that it speeds up or slows down (in a case where the reaction speed is explosive), the time at which a product will be formed from the reaction of the reactants and itself remains chemically unchanged.
In recent times, many industries, especially chemical industries and petroleum industries, make use of catalysts in large proportions. When industries first began using catalysts in their production activities, only pure components were used as catalysts. This changed in 1900 as research on multi-component catalysts developed. Industries now commonly use multi-component catalysts.
You can define industrial catalysts in two ways. They can be taken to mean the production of catalysts in large quantities for tjheir functionalities. You can also define industrial catalysts as the use of catalysts in industries such as the agrochemical industries, petroleum industries, and chemical industries to enhance the rate of reaction and selectivity of desired products.
Industrial catalysts are used because industries find catalysts useful in minimizing the harmful industrial waste generated during manufacturing processes, which can cause damage to the environment and become a threat to human health.
Industries also make use of catalysts to accelerate their chemical processes and thus get the best use out of their resources.
Even in humans, “enzyme”, a biological substance, helps in regulating the rate at which chemical reaction occurs in the body system, thereby serving as a catalyst.
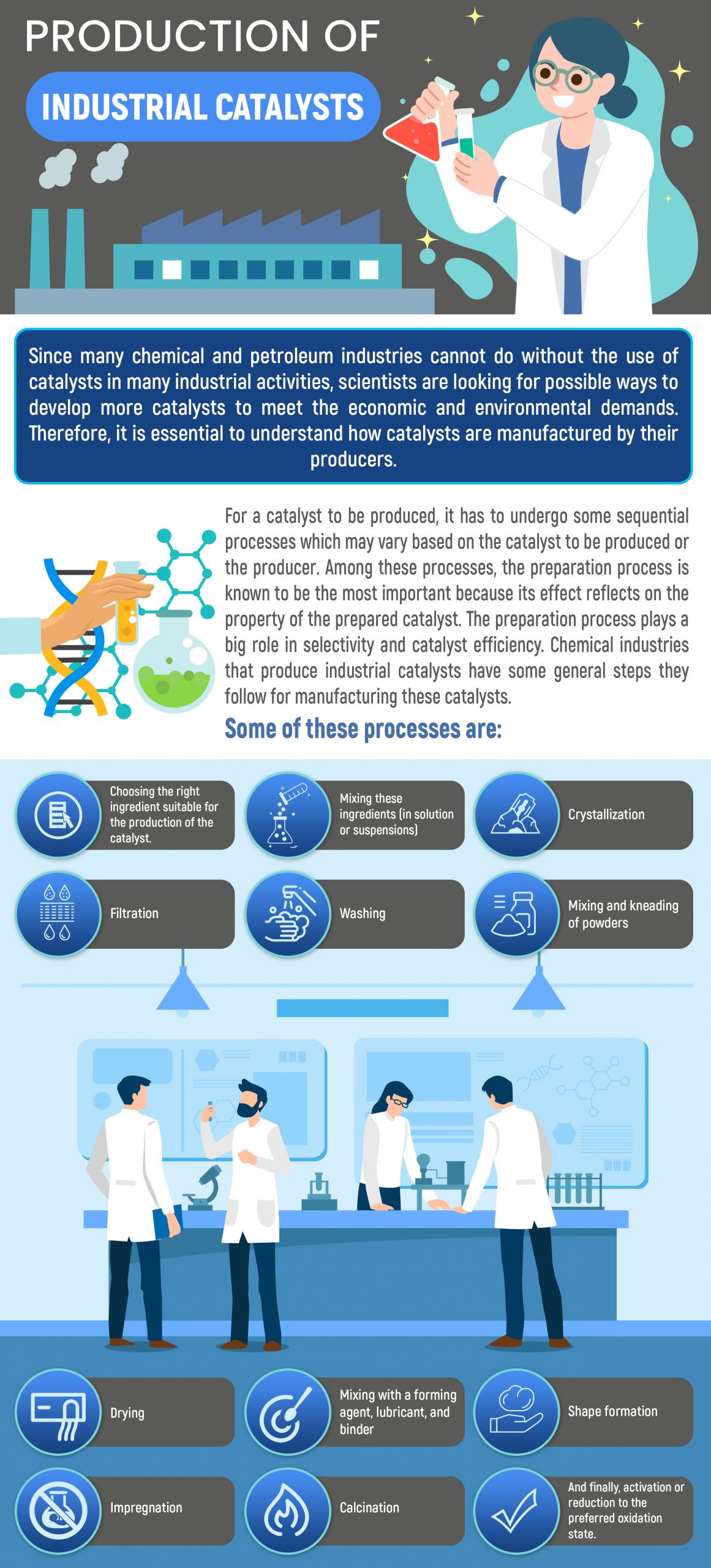
Production Of Industrial Catalysts
Since chemical and petroleum industries can't operate without the use of catalysts in most industrial activities, scientists are looking for ways to develop more catalysts to meet economic and environmental demands. It's therefore essential to understand how producers manufacture catalysts.
For a catalyst to be produced, it has to undergo some sequential processes which may vary based on the catalyst to be produced or the producer. Among these processes, the preparation process is known to be the most important because its effect reflects on the property of the prepared catalyst. The preparation process plays a big role in selectivity and catalyst efficiency.
Chemical industries that produce industrial catalysts have some general steps they follow for manufacturing these catalysts. Some of these processes are:
- Choosing the right ingredient suitable for the production of the catalyst
- Mixing these ingredients (in solution or suspensions)
- Crystallization
- Filtration
- Washing
- Mixing and kneading of powders
- Drying
- Mixing with a forming agent, lubricant, and binder
- Shape formation
- Impregnation
- Calcination
- Activation or reduction to the preferred oxidation state
The first thing to note during the process of producing industrial catalysts is to make sure that an active metal phase and support is selected. Support is the material that forms the largest component of the catalyst. The nature of the catalytic reaction in which the catalyst is present determines the activity of the support. Silica, zirconia, alumina, active carbons, magnesia, and zeolite are essential industrial supporters.
The support is made to be porous and heat resistant. The active metal component must also be well-dispersed in order to have a large surface area when contracted with the support.
After these, other procedures such as impregnation, drying, calcination, and filtration follow.
Industrial Application Of Catalysts
Catalysts are the fundamental support of many industrial processes that use a chemical reaction to process raw materials into useful products. Catalysts are used in the food production industries like breweries, beverage producers, and food producers, among others. Virtually everything in our daily life was made with the use of catalysts. They're used in the making of cars, soap, carbonated drinks, grilled chicken, polymers, glass, etc.
Catalysts are used in industries to break down pulp to produce sanitary paper, to turn milk into yogurt, and to refine crude oil into a series of end products, among countless other uses.
When a catalyst is used, a polluting chemical reaction can be reduced or replaced with an environment-friendly one. They are the driving forces used in making both biodegradable and non-biodegradable products available in today's world.
More reasons why catalysts can't be taken with levity in industries:
Imagine getting into a new car with great excitement, only to discover that the wheel is square-shaped. How hard would it be to drive the car? That is precisely how many chemical reactions would be without a catalyst. A catalyst is necessary to produce a well-formed finished product.
- Catalysts decrease the activation energy of a chemical reactions.
- Catalysts participate in reactions but are neither reactants nor products of the reaction they catalyze.
- They work by providing an alternative pathway for the reaction to occur, thus reducing the activation energy and increasing the reaction rate.
- Catalysts are not consumed in the process; they undergo various chemical changes during the process by interacting with the reactants and products.
- After reactions are complete, catalysts return to their original state.
- A minimal amount of catalyst can process many molecules.
Some common chemical catalysts used in industries are:
- Platinum + Alumina: A bifunctional catalyst (having two distinct functional groups).
- Nickel: A convenient heterogeneous catalyst used in the production of synthesis gas. It has a relatively low cost and serves as a better catalyst among other noble gases
- Iron: a catalyst commonly used for the Haber process (manufacture of ammonia from hydrogen and nitrogen)
- Vanadium: This is expected in the production of sulfuric acid.
- Aluminosilicate: This is an essential component in the modern petrochemical industry used to induce catalytic cracking (breaking up large hydrocarbon molecules into smaller bits in the presence of a catalyst to form a more useful product.
The Importance Of Catalysts In the Industry
The development of chemical products and the growth of technology in the world has made our society more industrialized. Gasoline, kerosene, and other petroleum products went through a series of industrial activities before they're used.
Catalysts are used in the production of polymers, clothing materials, fertilizers, chemicals, detergent, and many other products. Catalysts are also crucial in the production of preservatives used in fruits and vegetables and the fast ripening of fruits.
Virtually all industries use catalysts for at least one process. Industries that produce food, clothing, medicine, and many other products require catalysts as a critical substance in their production processes. With the aid of catalysts, products can be produced at lower costs; this makes high-quality products readily available for consumers at reasonable prices.
Although catalysts are expensive, they yield the desired products more quickly and accurately. The modern industrialized world would be inconceivable without catalysts. It's almost impossible to talk about production processes without taking the importance of Catalysts into account.
- They reduce production time
- They help in total and complete refining
- They save energy
- They help increase capacity
- They reduce waste
- They improve desired results
- They reduce the risks
- They suppress explosive reactions
Industrial Application of Homogeneous and Heterogeneous Catalysis
There are two main reactions:
- Homogeneous catalysis
- Heterogeneous catalysis
In a heterogeneous reaction, a catalyst is in a different phase from the reactant. While, in a homogeneous reaction, the catalyst is in the same phase as the reactant. This is in the sense that catalysts and reactants can either be in the same state (solid, liquid, or gas) or in the case of liquid if they are miscible or immiscible.
Catalysts can be further divided into:
- Positive catalysis
- Negative catalysis
- Acid catalysis
- Base catalysis
- Autocatalysis
- Enzyme catalysis (in the biochemical process)
Heterogeneous catalysis and Homogeneous catalysis are suited for different uses; they are chosen depending on the type of catalysts used.
Heterogeneous catalysis has a substantial number of advantages over homogeneous catalysis; as a result, most industries make use of heterogeneous catalysis. They play an important role in industrial chemical production. They are always preferred due to their easy separation qualities, and and they enables faster production, large-scale production, and selective production formation.
An example of heterogeneous catalysis is the hydrogenation of a carbon-carbon double bond. Other examples of industrial processes using heterogeneous catalysts are:
- Sulfuric acid synthesis (contact process)
- Desulfurization of petroleum (hydrodesulfurization)
- Reduction of nitrites in the synthesis of phenethylamine with Raney nickel catalyst and hydrogen in ammonia
An example of homogeneous catalysis is the reaction between per phosphate ions and iodide ions. Enzymes are also a homogeneous catalyst that help in the process of human life. Examples of industrial processes using homogeneous catalyst are:
- Transition metal catalysis
- Carbonylation (it can be in the conversion of alcohol to carboxylic acids)
- Polymerization and metathesis of alkenes
The Role of Catalysts in the Desulfurization Process of Crude Oil
Desulfurization is removing sulfur from crude oil. The essential characteristics of crude oil that affect the market price are its sulfur content and gravity. Low sulfur content of crude oil means higher quality and a higher price.
The emission of sulfur dioxide (SO2) from fuels when burned leads to air pollution. As a result, a government-specified level to which sulfur must be reduced in each country must be met by refineries.
There are different processes and methods of desulfurization developed by various research institutes and refineries over the past decades. However, HDS, Hydrodesulfurization process is the most common method of removing sulfur from crude oil at the industrial scale.
HYDRODESULFURIZATION
HDS is the most common technology used by refineries to remove sulfur from crude oil and its distillates (gasoline, kerosene, and diesel oil).
It's a technology that converts organic sulfur compounds to hydrogen sulfide under:
• High temperature (290–455°C; 555 to 850°F); and
• High pressure (150–3000 psi)
It uses hydrogen gas in the presence of metal catalysts, e.g., CoMo/Al2O3 or NiMo/Al2O3, to convert the organosulfur in crude oil into hydrogen sulfide.
The produced hydrogen sulfide is then catalytically air-oxidized to elemental sulfur.
At large scales, the most economical technology for converting hydrogen sulfide into elemental sulfur is the Claus process. This well-established process uses partial combustion and catalytic oxidation to convert about 97% of the H2S to elemental sulfur.
Catalysts play an important role in the HDS desulfurization process of crude oil. The HDS catalysts include molybdenum and tungsten with nickel or cobalt promoters.
The Function of Catalysts in the Hydrodesulfurization Process of Crude Oil
The catalysts used in HDS are materials with a high surface area. They provide a wide interfacial area between the liquid and gaseous phases when the reaction occurs in a slurry reactor.
They contain an active ingredient and an accelerator that is distributed on a base (alumina). The primary catalysts are cobalt–molybdenum and nickel–molybdenum supported on alumina.
Alumina has outstanding textural and mechanical properties and it is relatively cost-effective. The Alumina support is used principally to achieve better catalyst dispersion, which consequently increases the catalytic performance.
Cobalt-molybdenum catalyst has a low amount of hydrogenation activity and consequently has a low consumption of hydrogen for sulfur removal.
BENEFITS OF CATALYSTS IN THE ENVIRONMENT
Catalysts are known for their ability to speed up the rate of a chemical reaction. This alone is a great benefit to the environment. Some reactions will take years if a catalyst is not present. The presence of catalysts in many reactions has saved a lot of time and money.
Here are some other benefits of catalysts to our environment:
- They help save energy and produce more with less work
- They can be reused multiple times till they become spent
- They help facilitate the production of a better product
- They reduce the temperature at which reactions occur
In the instance of silver catalysts, they produce fewer toxic by-products, and that alone makes the whole reaction more environmentally friendly. A catalyst saves energy, and applying catalysts on a large scale could save the world a lot of energy, ranging from edibles to other hazardous wastes and metals.
Catalysts provide environmental benefits of clean air by reducing pollutants produced from stationary and mobile combustion sources. Even fast-food restaurants use catalysts to remove odors from the cooking process.
Real-World Usage of Industrial Catalysis
Catalysis refers to the addition of catalysts in order to bring about an increase or decrease in the rate of a chemical reaction as the case may be. A significant percentage of all the industrial products, chemicals, and materials produced worldwide involve the use of catalysts.
Petroleum industries are a giant consumer of catalysts because they have four important processes that yield results. These four processes are:
- Catalytic reforming: This is an important process in the petroleum refinery. It is the chemical process of converting petroleum refinery naphtha distilled from crude oil into reformates which have a higher octane number in the presence of a platinum-containing catalyst.
- Hydrotreating: This is also known as the hydrogen process. This is the most widely used method for the removal of sulfur and nitrogen impurities from the oil. This is done by mixing the oil with highly purified hydrogen and then vaporizing it before it is passed over catalysts such as nickel or tungsten. This is usually done at a temperature ranging from 260-425oC. Hydrotreating is generally used for the purpose of improving product quality without appreciable alteration of the boiling range.
- Catalytic cracking: This is a process by which high molecular weight hydrocarbons are converted into more valuable gasoline, olefin, and other useful products in the presence of a catalyst under a suitable condition of temperature and pressure. It's a thermal decomposition of heavy petroleum hydrocarbon in the presence of a catalyst. It's an ionic process involving carbonium ions, it gives higher yield and higher-octane number.
- Alkylation: This is a process whereby light hydrocarbons such as propene, butene, and isobutane are combined with the aid of a catalyst to produce high-octane petrol. It can also refer to the process of producing high-octane motor fuel components by the combination of olefins and with a tertiary carbon atom.
Catalytic cracking uses three different catalysts:
- Primary catalyst: this can be natural or artificial clay, an example is a bentonite.
- Metallic catalyst: examples of this can be platinum, chromium, iron, and nickel. They are easily poisoned by sulfur and arsenic-containing compounds.
- Synthetic catalyst: examples of this include silica-alumina and silica-magnesia.
There are three types of catalytic cracking process:
- Fixed bed
- Moving bed
- Fluidized bed

How Does A Catalyst Become Spent?
Catalysts gradually lose their catalytic ability when saturated by metal, sulfur, or halides or contaminated with impurities. They lose their surface area from sintering at high temperatures. They can also lose their activity through overheating, structural changes, or coking. A catalyst is “spent” when it no longer exhibits the necessary activities that are required of it in a reaction, as they get reduced in both the chemical composition and in its physical properties. High metal concentration may also cause spent catalysts.
Some other mechanism catalysts become “spent” because of the following during the reaction process:
- Sintering
- Vapor-solid reaction
- Coking
- Fouling
- Solid-state transformation
- Erosion
Catalysts can often be used for years before they become spent. They can be used and reused for different reactions.
CHARACTERISTICS OF SPENT CATALYSTS
The hazardous characteristics spent catalysts are toxic in nature. Spent catalysts decrease the reaction rate and any substances that decrease the reaction rate are poison. Poison accumulates on the surface of a catalyst and reduces the number of available active metals for reactant molecules to bind to. Elements that expend catalysts are, sulfur, oxygen, phosphorus, chlorine, and some toxic metals such as Arsenic, Lead, etc.
Catalysts exhibit the following characteristics when they are deactivated (spent):
- They decrease the rate of a chemical reactions
- They poison the reactants
- They do not lower the activation energy
Handling of Spent Catalysts
Industries that produce spent catalysts can't handle them alone. Their toxic nature to the environment and human health makes them tough to handle. Because of this, environmental authorities have given strict regulations guiding their disposal. This makes it difficult for companies to find safe ways of handling spent catalysts.
Handling spent catalysts requires certain specialization and facilities to avoid getting sanctioned by law. Fortunately, registered and certified companies like Recognized Trading and Shipping Inc., and partners deal with the transporting and recycling of these spent catalysts in a legally compliant way.
Recycling has been the best way so far to achieve safe disposal of spent catalysts. The recycling of spent catalysts requires specific steps, such as:
- De-oiling
- Drying
- Grinding
- Sieving
- Decoking
The recycling process has been a significant move in reducing hazardous wastes disposal and saving natural resources with economic benefits. The spent catalyst recovery process is not only an economical process that recovers metals but it's also a very important option for waste recycling and re-utilization.
Recovery of Metals From Spent Catalysts
Catalysts are spent when they have been contaminated by impurities. When this happens, they can't serve the purpose of a catalyst any longer. Therefore, they have to be disposed of in an environmentally friendly way or treated before being reused.
If spent catalysts are not properly handled, they may become hazardous to our environment and threaten lives.
Spent catalysts have been categorized as hazardous waste because they may contain poisonous elements like sulfur, phosphorus and lead. They're composed of poisonous metals, they also contain precious metals like platinum, palladium, and rhodium which could be used by other industries to produce useful products such as jewelry and electronics.
Spent catalysts comprises many other metals that can in turn be useful when recovered, depending on original components. Metals that are recovered from spent catalysts could be; molybdenum, vanadium, nickel, cobalt, etc.
Depending on the metal to be recovered from a spent catalyst, different approaches are used in the recovery of precious metals from the spent catalyst.
SPENT CATALYSTS ARE USEFUL
Instead of wrongly disposing of spent catalysts in a way that pollutes our environment and may attract legal penalties, they can and should be propertly recycled into useful components.
Here are some ways that catalysts are useful:
- Spent catalysts are useful for the preparation of useful materials like electronics, building materials, etc.
- Active metals like molybdenum and nickel can be extracted and reused
- They can be regenerated and reused for their original purpose
- Some or all of the components in the material can be recovered
- Some metals extracted from it can be used to make valuable assets like jewelry and electronics
- Catalysts often contain titanium oxide and metals such as Tungsten and Vanadium in appreciable concentrations and thus could be used as a secondary source for these valuable metals
- Recovered metals such as molybdenum, vanadium, nickel, and cobalt could be used in steel manufacturing and the alumina could be used for the manufacture of refractories, ceramics, and abrasives

The Best Ways to Manage Spent Catalysts
The rate at which the petroleum refining industry produces spent hydroprocessing catalysts is overwhelming , and this is due to the rapid growth in the hydroprocessing capacity to meet the rising demand for low sulfur fuels. Spent catalysts are classified as hazardous waste, because of their toxic nature. Many chemical processing industries across the world produce a large number of spent catalysts, but the major proportion of spent catalysts come from the hydroprocessing units of petroleum refineries.
Because of the harmful effect of these industrial spent catalysts on human health, there has been an increase in environmental awareness by concerned authorities to prevent the hazard caused by these harmful wastes generated by industries.
Spent catalysts can be very dangerous when improperly handled or stored in a place for a long period. When spent catalysts are improperly stored, they can explode and destroy an entire community and it can ruin agricultural lands, oceans, and groundwater.
STEP BY STEP PROCESS OF RECYCLING SPENT INDUSTRIAL CATALYSTS
There are four primary steps involved in the recycling and reprocessing of spent industrial catalysts.
They are:
1) Pre-Kiln Screening
2) Rotary Kiln Firing
3) Pollution Prevention
4) Production / Reprocessing
Recognized Trading & Shipping specializes in facilitating the recycling of spent catalysts by shipping them to worldwide destinations for proper treatment and recovery. For more information, call (213) 444-9966.



